LyGenesis has pioneered an innovative experimental therapy with the potential to transform the landscape of medical treatment by growing miniature livers within the lymph nodes of patients.
This groundbreaking approach offers a promising solution to the challenges posed by severe liver disease and the acute shortage of organ donors.
The experimental therapy, referred to as LYG-LIV-001, involves the transplantation of hepatocytes, which are specialized liver cells obtained from donated livers, into the patient’s lymph nodes.
The hypothesis behind this treatment is that these transplanted liver cells will proliferate and mature over time, eventually developing into fully functional liver tissue within the patient’s body.
The primary objective of this innovative therapy is to offer a new and effective treatment option for individuals suffering from end-stage liver disease (ESLD). ESLD is a severe and often life-threatening condition that affects millions of people globally each year.
The shortage of suitable liver donors has resulted in long waiting lists for liver transplants, leading to a critical need for alternative treatment options.
LyGenesis’ therapy could potentially fill this gap by utilizing a patient’s own body to grow a new liver, which could save many lives and significantly improve patient care.
While the outcome of the ongoing clinical trials remains uncertain, LyGenesis sees a broader potential for this groundbreaking technology beyond treating liver disease.
The company envisions that this innovative approach could be adapted to cultivate tissues for other vital organs, such as kidneys, thymus, and pancreas.
If successful, this could pave the way for transformative developments in the field of regenerative medicine, offering new hope to patients suffering from a variety of organ-related conditions.
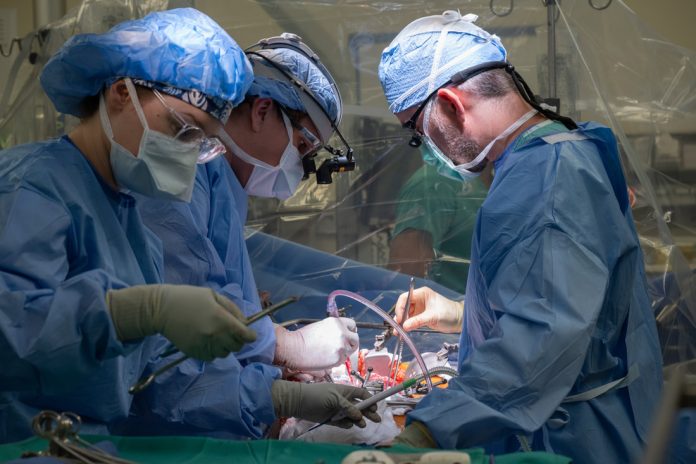
LyGenesis has taken a significant step forward in the development of this innovative therapy by initiating human trials. In March, they began injecting the experimental treatment into 12 patients diagnosed with end-stage liver disease.
Dr. Michael Hufford, the Co-Founder and CEO of LyGenesis, has expressed optimism about the potential of this groundbreaking therapy.
He said, “If our study is successful and we obtain FDA approval, our allogenic cell therapy could enable one donated liver to treat many dozens of ESLD patients.
This could help to address the current imbalance between the supply and demand of organ donors, benefiting patients in need.”
Liver disease is a major public health concern, with millions of people diagnosed globally each year and over 50,000 people dying from it. Currently, nearly 10,000 individuals are on waiting lists for a liver transplant, and the availability of suitable donated livers is limited.
The significant advantages of LyGenesis’ new therapy is that it requires only a small number of liver cells from a single donor. This means that each donated liver could potentially provide enough material to treat up to 75 patients.
This innovative approach could significantly increase the number of people who can benefit from a single donated organ, making a substantial difference in the lives of those awaiting a life-saving liver transplant.