New Delhi, After at least 70 children died last year after drinking cough syrup in the West African country of Gambia, the government has tightened rules for imported medicines from India.
In this regard, the special report of the foreign news agency Reuters has stated that the Gambia will make inspection and testing of all pharmaceutical products imported from India mandatory from July 1.
In Gambia, 70 children were allegedly killed by cough syrup produced by an Indian company. The presence of this toxic solvent in several brands of syrups has raised serious questions about the drug regulatory system in India.
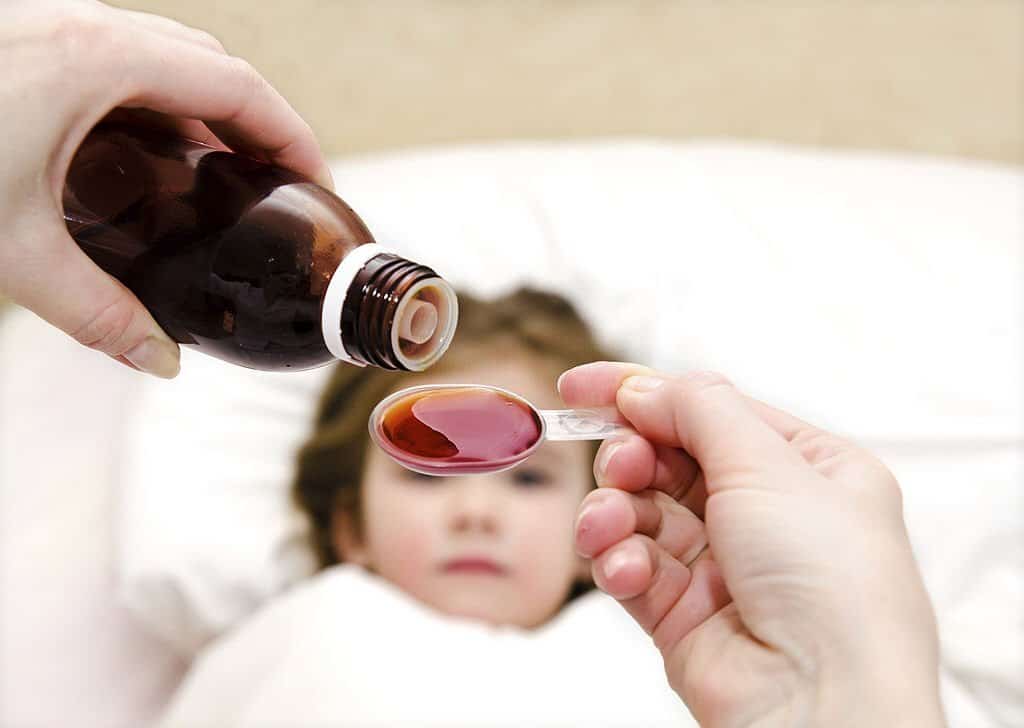
According to a Reuters investigative report, Gambia’s government documents show that the export ban is the first since dozens of children died from an Indian-made cough syrup made by Indian drugmaker Marine Biotech.
The new rules indicate that governments in several countries are reassessing their reliance on India’s $42 billion pharmaceutical industry following the decision by the Gambian government. It should be noted that Indian pharmaceutical companies supply almost half of the drugs used in Africa.
The Executive Director of Gambia’s Medicines Control Agency, Markio Jania Kaira, has sent a letter to the Drug Controller General of India, Rajeev Singh, stating that our initiative is aimed at addressing the issue of substandard and counterfeit drugs being imported into the country.
18 children also died in Uzbekistan due to drinking Indian medicine
Last year, at least 70 children, most of them under the age of 5, died of acute kidney disease in The Gambia, which doctors attributed to adulterated cough drops imported from India. The syrup was declared the cause of death.


![[Downloader.la]-64946e5795c1d-min (1)](https://startuppakistan.com.pk/wp-content/uploads/2023/06/Downloader.la-64946e5795c1d-min-1-696x495.jpg)