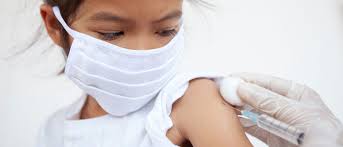
The Emergency Use Approval (EUA) for the second COVID-19 vaccine to be used in emergencies throughout the country has been issued by the Drug Regulatory Authority of Pakistan (DRAP).
According to the information, DRAP approved the coronavirus vaccine produced for emergency use in the country by the state-owned China National Pharmaceutical Group, known as Sinopharm.
A spokesperson for DRAP has said that the regulator has approved two coronavirus vaccines for emergency use across the country after determining their safety and efficacy.
In a recent interview, Dr. Faisal Sultan, said that Pakistan will soon procure different COVID-19 vaccines both directly and thru the COVAX facility.
Similarly, Asad Umar, Federal Minister for Planning, revealed that the govt has approved the procurement of the COVID-19 vaccine to be scheduled for immediate deployment within the country by March 2021.